
Basant
Basant is a clinically proven polyherbal formulation which prevents the entry of HPV-16 in cervical cells, and is proven to be effective in eliminating HPV-16 from infected cervical cells on path of progression to carcinoma of Cervix.
Basant prevents sexually transmitted infections: Gonorrhea, Chlamydia, Candida, H.I.V.

Clinical results
- Basant inhibits the entry of HPV 16 in Hela cells as observed by Dr. Schiller at NIH, Bethesda [1].
- Basant eliminates HPV16 from infected cervical cells on path of progression to carcinoma of Cervix.(1)
- Basant has inhibitory action on a wide spectrum of genital pathogens: Neisseria gonorrhoeae, all strains of WHO and those resistant to various antibiotics such as Penicillin, Tetracycline, Nalidixic acid and Ciprofloxacin [1].
- It has pronounced inhibitory action against Candida glabrata, Candida albicans and Candida tropicalis isolated from women with vulvovaginal candidiasis, including three isolates resistant to azole drugs and amphotericin [3].
- Basant inhibits Chlamydia trachomatis, whether present in free-state or within a cell [3]. It is highly effective for treatment of recurring episodes of Vaginosis and restoring healthy vagina.
Composition
Basant is composed of Standardised Curcuminoides, extracts of Amla (Emblica officinalis), Aloe vera (Aloe barbadensis) and Azadirachta indica (Neem) leaves along with pharmacopoeially approved excipients


Packing – 30 capsules
Basant is packaged in easily dispersing cellulose capsules, which can be intra vaginally administered with ease . These are fully safe and non-irritating
BASANT exercises both Preventive and Therapeutic action against Human Papilloma Virus (HPV)
HPV-16 and HPV-18 are amongst the most dominant strains of HPV, which lead to carcinoma of cervix, the major reproductive tract cancer of women. John Schiller at the National Cancer Institute, NIH, Bethesda USA observed that 2 ingredients of BASANT namely Amla (Emblica officinalis) and Aloe vera (Aloe barbadensis) inhibit the transduction of HPV-16 in Hela cells at concentrations far below those that are cytotoxic and those used in the formulation [1]. BASANT inhibits the entry of the highly pathogenic strain of HPV-16 in Hela Cells . What is further amazing is that BASANT eliminates HPV-16 from infected cervical cells on path of progression to carcinoma of cervix till the stage that virus is not yet integrated into the host genome. The virus is expelled and Pap smear is rendered normal by 30-night intravaginal administration of BASANT capsule [5].
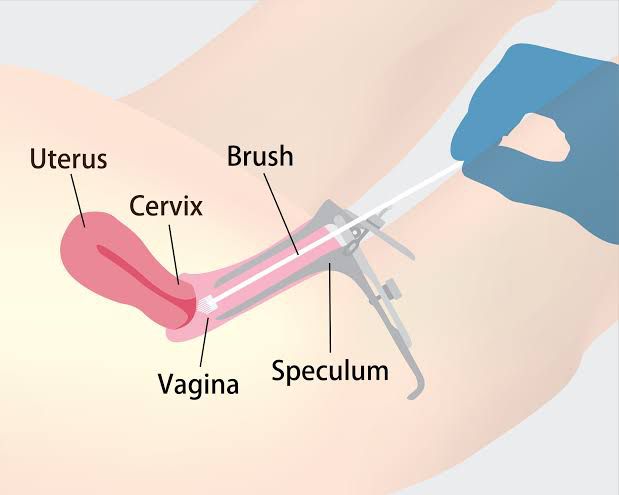
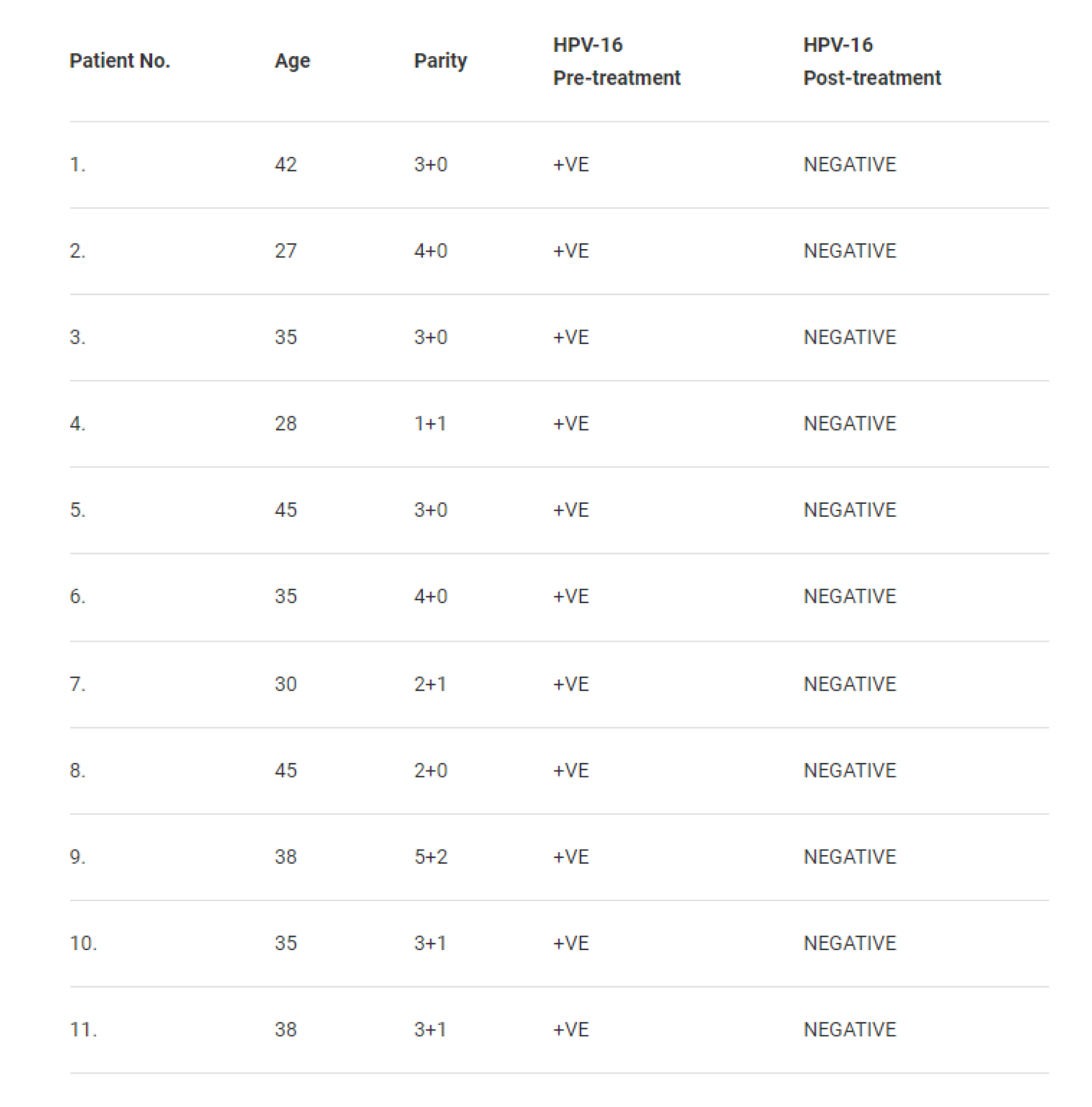
A clinical trial was conducted at the Jawaharlal Nehru Medical College Aligarh, Uttar Pradesh along with the Institute of Cytology and Preventive Oncology of the Indian Council of Medical Research. One hundred fifty nine women coming for various types of treatment to the Obstetrics and Gynaecology Clinic were examined clinically by visual inspection of cervix and by staining with acetic acid. Cervical scraps were collected from the ectocervical region and endo-cervical canal to prepare Pap smears. 35 Women who had inflammatory cervix and abnormal Pap smear were enrolled. Out of these 19 women were positive for HPV-16. Eleven of these women gave written consent to undergo intravaginal treatment with BASANT. They were advised to insert a capsule of BASANT in the vagina every night before going to bed for 30 days continues, excluding the days of menstruation. Table 1 given below summarizes the observations.
Most amazing was the observation that every woman (11/11) who used BASANT had no HPV-16 in their cervical cells. Pap smear was rendered normal in each case.
Table 1: Pre and post treatment with BASANT of HPV-16 positive patients [5]
References
- Talwar GP, Dar SA, Rai MK, Reddy KV, Mitra D, et al. (2008) A novel Polyherbal microbicide with inhibitory effect on bacterial, fungal and viral genital pathogens. Int J Antimicrob Agents 32(2): 180-185.
- Garg KB, Ganguli I, Ram Das, Kriplani A, Lohiya NK, et al. (2009) Metabolic properties of lactobacilli in women experiencing recurring episodes of bacterial vaginosis with vaginal pH≥5. Eur J clin Microbiol Infect Dis 29(1): 123-125.
- Bhengraj AR, Dar SA, Talwar GP, Mittal A (2008) Potential of a novel polyherbal formulation BASANT for prevention of Chlamydia trachomatis infection. Int J Antimicrob Agents 32(1): 84-88.
- Maselko MB, Joshic RS, Prescott M, Talwar GP, Kulkarni S, et al. (2014) Basant, a polyherbal topical microbicide candidate inhibits different clades of both ccr5 and cxcr4 tropic, lab-adapted and primary isolates of human immunodeficiency virus-1 in vitro infection. J Virol Antivir Res 3(4): 3.
- Talwar GP, Sharma R, Singh S, Das BC, Bharti AC and Sharma K, et al. BASANT, a Polyherbal Safe Microbicide Eliminates HPV-16 in Women with Early Cervical Intraepithelial Lesions. Journal of Cancer Therapy. 2015;6:1163-1166. Doi: 10.4236/jct.2015.614126.
- Bhujwala RA, Buckshee K and Shriniwas. Gardnerella Vaginitis and associated aerobic bacterial in nonspecific Vaginitis. Ind J Med Res. 1985;81: 251-256.
- Bang RA, Bang AT, Baitule M, Sarmukaddam S, Choudhary Y and Tale O. High prevalence of gynecological diseases in rural Indian women. The Lancet. 1989;1(8629):85-88.
- Cudmore SL, Delgaty KL, Hayward-McClelland SF, Petrin DP and Garber GE. Treatment of infections caused by Metronidazole-resistant Trichomonas vaginalis. Clin Microbiol Rev. 2004;17(4):783–793.
- Talwar GP, Kavita G, Atrey N, Singh P, Gaur J and Jagdish CG, et al. A Safe Wide Spectrum Polyherbal Microbicide and Three Meritorious Strains of Probiotics for Regressing Infections and Restoration of Vaginal Health (Regression of Vaginosis with BASANT and Probiotics). J Women’s Health Care. 2015;4(5):256. Doi:10.4172/2167-0420.1000256.







